EN ISO 18562: Biocompatibility Evaluation of Breathing Gas Pathways in Healthcare Applications
The title of the EN ISO 18562 family of standards is “Biocompatibility evaluation of breathing gas pathways in healthcare applications”. It consists of four parts, which have been harmonized under the EU directives since 2020.
This article will help you to implement these standards, e.g., when determining which tests you actually have to carry out for your medical device. With the information and tips given here, you will be able to reduce time and costs to a minimum and avoid several problems that regularly occur in practice.
1. Problems when implementing EN ISO 18562
In practice, there are often problems when it comes to the implementation of the EN ISO 18562 family of standards:
- A lot of manufacturers are still unfamiliar with this family of standards. Others don’t know when this standard is applicable and how it differs from ISO 10993.
- The new approaches to release kinetics, in particular the TTC/AET approaches, sometimes require complex test designs.
- The tests are (also because of this) often very cost intensive.
- Only a few laboratories offer the corresponding tests.
- The applicability of the standard is not always clear.
This article gives advice on how to avoid these problems.
2. Scope of EN ISO 18562
a) Difference from the EN ISO 10993 family of standards
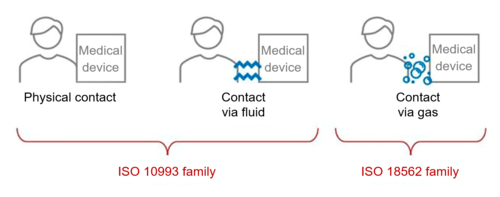
The EN ISO 10993 series ”Biological evaluation of medical devices” applies when determining the biocompatibility of medical devices that come into direct contact with patients.
However, if a medical device does not come into direct contact with patients, or only comes into indirect contact with patients via fluids, EN ISO 10993 is not applicable. This is the case, for example, for medical devices that come into indirect contact with the respiratory tract due to being gas pathways.
EN ISO 10993-1 refers to the device-specific standard.
“For gas pathway device components with only indirect contact, device specific standards should be used to determine the relevant type of biocompatibility evaluations [see ISO 18562 (all parts)].”
EN ISO 10993-1, 5.1
Definition: gas pathway
“interior surfaces, over which gases or liquids that can be inspired, in a medical device bounded by the ports through which gases or liquids enter and leave the medical device including the patient interface or the interior surfaces of enclosures that are in contact with gases or liquids that can be inspired”
EN ISO 18562, 3.5
b) Examples
For extra clarity, EN ISO 18562-1 gives several examples of devices that fall under the scope of the standard:
- Therapeutic medical devices
- Ventilators
- Respiratory gas monitors
- Anesthesia workstations
- Incubators
- Gas mixers
- Nebulizers
- Humidifiers
- Breathing system filters
- Oxygen concentrators
- Gas pathways
- Y-pieces
- Breathing tubes
- Masks
- Oxygen conservation equipment
- Low-pressure hoses
c) Further help
Despite the definitions and examples, manufacturers and notified bodies don’t always agree. The Johner Institute, therefore, recommends the following rule of thumb:
ISO 18562 applies irrespective of the size of the medical device if the patient actively inhales air or gas through the device.
NB!
If EN ISO 18562 applies to your medical device and your device also comes into direct contact with patients, then EN ISO 10993 may also be applicable. But with a bit of luck, the test results for one standard can be transferred to the other. You can read how to do this further down this article.
3. The EN ISO 18562 family of standards
a) Overview
The title of the series of standards is “Biocompatibility evaluation of breathing gas pathways in healthcare applications”. There are four parts.
The first should be viewed as an overarching part that creates the framework for the series and is always applicable.
Parts 2 to 4 look at specific issues and are not applicable to every medical device that falls within the scope of ISO 18562.
Standard | Title | Applicable |
ISO 18562-1 | Evaluation and testing within a risk management process | Always; general information |
ISO 18562-2 | Tests for emissions of particulate matter | In cases of possible suspended matter, e.g., due to device movements |
ISO 18562-3 | Tests for emissions of volatile organic compounds (VOCs) | In cases of possible volatile substances, e.g., certain plastics |
ISO 18562-4 | Tests for leachables in condensate | In cases of possible condensates that can flow into the patient |
Just like the new EN ISO 10993-1, EN ISO 18562 gives absolute priority to material characterization and in vitro testing over animal testing. This is another concrete milestone on the path to animal-free testing of medical devices.
The Johner Institute assumes that standards will increasingly follow this testing strategy in the future.
You can purchase the standards in the EN ISO 18562 series, from Beuth-Verlag for example.
b) EN ISO 18562-1: Evaluation and testing within a risk management process
EN ISO 18562-1 primarily describes the integration of the risk management process into the planning and evaluation of biocompatibility. This process should conform with EN ISO 14971. ISO 14971 and TR ISO 24971 (German) provide guidance on the benefit-risk analysis.
This first part of the EN ISO 18562 family of standards:
- establishes the essential requirements
- gives an overview of the other parts of the series
- defines the terms
- provides guidance on test selection
- provides guidance on biocompatibility planning and evaluation
- also provides information on how to handle substances released by the medical device.
According to ISO 18562-1, the following aspects must be taken into account when evaluating biocompatibility:
- Materials
- Additives
- Process contaminants and residues
- Substances released during use
- Degradation products and material interactions
These requirements make it clear once again that an assessment of biocompatibility based solely on material and data sheets is not appropriate and, as a rule, is not possible.
Similarly, for reusable medical devices, EN ISO 18562 requires consideration of the effect of all reprocessing steps. This requirement is also established in the MDR and EN ISO 10993.
Additional information
The following articles will help you
- understand why biocompatibility is very difficult to prove on the basis of materials and data sheets alone
choose the right reprocessing steps for reusable medical devices
c) EN ISO 18562-2: Tests for emissions of particulate matter
EN ISO 18562-2 contains specific and comprehensive guidance on testing for particulates. You can find out whether this second part is applicable for your device simply by reading what the standard says:
“A simple component such as a connector with minimal area exposed to the patient breathing gas stream is very unlikely to need testing for particulate matter, while a mechanical medical device with moving parts such as a ventilator could well require thorough testing.”
EN ISO 18564-2, 5.2
In principle, the emission of particles does not refer to impurities from production, but particles that are generated by the operation of the medical device itself (its parts or accessories) and that can enter the gas flow.
Definition: particulate matter (pm), particulates
“solid particles suspended in a gas”
EN ISO 18562-1, 3.10
In this case, all particles between 0.2 and 10 µg in size must be quantified. The standard sets specific limit values according to particle size:
Particle size [µm] | Maximum particulate quantity [µg/m3] |
< 0.2 | Standard not applicable (nanoparticles) |
0.2 to 2.5 | 12 |
2.5 to 10 | 150 |
> 10 | Standard not applicable |
Particles with dimensions below 0.2 µm are considered nanoparticles. The standard does not apply to these particles. You should refer to ISO/TR 10993-22 “Guidance on nanomaterials” instead.
d) EN ISO 18562-3: Tests for emissions of volatile organic compounds (VOCs)
Scope
For medical devices that come into contact with patients via respiratory gases, the release of volatile substances into the gas is important.
It is understandable that high quantities of volatile substances (e.g., solvents) can harm patients. Therefore, it is important to rule out the release of volatile substances or to prove their quantity.
A distinction is made between:
- VOCs: volatile organic compounds
- VVOCs: very volatile organic compounds
Definition: very volatile organic compound (VVOC)
“organic compound whose boiling point is in the range of 0 °C to 50 °C”
EN ISO 18562-1, 3.17
Definition: volatile organic compound (VOC)
“organic compound whose boiling point is in the range of 50 °C to 260 °C”
EN ISO 18562-1, 3.16
Officially, EN ISO 18562-3 only requires the detection of possible VOCs.
But be careful:
“Some AUTHORITIES HAVING JURISDICTION require evaluation of SVOCs and VVOCs.”
EN ISO 18562-1, 5.3
Requirements
For a lot of substances, there are toxicological limits that help evaluate released volatile substances.
The TTC (threshold of toxicological concern) approach is used to ensure that substances above a certain quantity are actually detectable with the analyses performed and are detected.
Definition: threshold of toxicological concern (TTC)
“level of exposure for all chemicals, known or unknown, below which it is considered there is no appreciable risk to human health”
EN ISO 186562, 3.12
This means you can ensure that analytically undetectable quantities (because they are too low) of an unknown substance do not pose a toxicological risk.
However, as even smaller quantities of a critical substance can cause problems over a prolonged period, the TTC decreases continuously over the duration of contact. In short:
A larger quantity of released substances over a short period of time is just as critical as a very small quantity coming into contact with the patient over a long period of time.
Exposure duration in days (d) | TTC [µg/d] |
? 1 | 360 |
1–30 | 120 |
> 30 | 40 |
Tip
The test setup and the test specimen selection are vital for a successful test. They ensure that the results for your medical device are neither underestimated or unnecessarily overestimated. It is always best to test the VVOC at the same time. Depending on the laboratory, this may not involve any additional costs and you close any technical gaps on issues related to volatile substances.
e) EN ISO 18562-4: Tests for leachables in condensate
If condensates form in the gas pathways and can flow back to the patient, the condensates must be tested for possible leachables. Condensates can either be extracted or produced (by extraction) by the company itself for these tests.
Definition: leachables
“leachable substance chemical removed from a medical device by the action of water, other liquids or other gases (e.g. anesthetic agents or inhalational drugs) related to the use of the medical device EXAMPLE Additives, sterilant residues, process residues, degradation products, solvents, plasticizers, lubricants, catalysts, stabilizers, anti-oxidants, coloring agents, fillers and monomers, among others.”
ISO 18562-1, 3.6
In addition to the organic components, (depending on the material) condensates must also be tested for possible inorganic substances (testing, e.g., according to USP <233>). All components that can come into contact with condensate are affected.
In this case, you also have to perform a cytotoxicity test according to EN ISO 10993-5. In addition, make sure you select the best laboratory to avoid false negative results.
The standard suggests testing condensates for sensitizing substances according to ISO 10993-10. Therefore, if substances are detected, the specifications and guidance in EN ISO 10993-18/-17 can be used in addition to that of EN ISO 18562, e.g., to calculate the “margin of safety”.
Tip
“Only sections of the gas pathway from which the patient can be exposed to condensate need be tested.”
EN ISO 18562-1, 5.4
4. Six tips for minimizing costs and time
The following tips can help you reduce the costs and the overall time required for testing. Due to the technical complexity, we have been very concise with these tips. If you would like more detailed help, just contact us.
Tip 1: make a worst-case selection
You don’t have to test the complete product range. Use the following characteristics to select critical products:
- Materials
- Material ratios
- Absolute surface areas
- Adhesives
- Geometry
However, experience has taught us that there are usually several worst-case devices.
Tip 2: use the results of other tests
If you have test results from the final cleaning, from EN ISO 10993 testing, or test data from suppliers, with a bit luck you will be able to transfer these results.
The tests for volatile substances and questions about extractables (dissolved substances in condensates) are often already technically covered by ISO 10993-18 tests. This is particularly the case if you have already tested the component because it comes into direct contact with patients.
You may also be able to draw enough conclusions about your current device from the testing of other devices in your portfolio. It’s definitely worth taking a closer look.
Tip
“If the medical device under evaluation has already been evaluated as tissue contacting according to ISO 10993-1, then leachable substances tests need not be performed in addition.”
EN ISO 18562-1, 5.4
Tip 3: use type tests
Is it difficult to test the final device? Then use type tests. Complicated test designs drive test costs up unnecessarily.
Definition: type test
“test on a representative sample of the MEDICAL DEVICE with the objective of determining if the MEDICAL DEVICE, as designed and manufactured, can meet the requirements of this document”
EN ISO 18562-1, 3.15
Tip 4: avoid making changes
Try to avoid unnecessary changes to your device and manufacturing process as much as possible. If changes are made to any of the following, you’ll have to re-evaluate biocompatibility:
- Recipe
- Color
- Processing
- Intended use
- Supplier
- Manufacturing
- Adhesives
Tip 5: monitor the test results
A lot of manufacturers don’t have much experience with these “new” tests. This particularly relates to the assessment of
- the plausibility of released substances and quantities and
- the toxicological effects if such substances are detected
Keep an eye on your test results right from the start, both the material tests and device tests. Plausibility checks and estimates of quantities of released substances based on test history are worth hard cash and save you significant test costs.
Tip 6: be prepared for unclear requirements from notified bodies
Unfortunately, we have seen on several occasions that some notified bodies do not approach the specifications of EN ISO 18562 uniformly, even for the same devices. This is unfortunate, but understandable, as there are a lot of special cases that leave room for interpretation.
So, also be prepared for demands relating to section 4.5 of EN ISO 18562-1:
- Ozone, for gas pathways in contact with active electromechanical or electrostatic parts in normal condition
- CO and CO2, for gas pathways where inorganic gases are generated or concentrated
- Leachables, for gas pathways in contact with anesthetic agents where the gas can be inspired in normal condition
- Leachables, for gas pathways in contact with substances intended to be delivered via the respiratory tract (e.g., inhalational drugs)
5. Conclusion
The EN ISO 18562 family of standards helps you demonstrate compliance with the MDR requirements on the biocompatibility of breathing gas pathways in healthcare applications.
The standard has four parts. You should, first of all, work out which parts of this family of standards that apply in your case.
You can save a significant amount of time and money through:
- A smart worst-case selection
- Transferring test results that you have generated in another context
If, contrary to expectations, your medical device gives abnormal results or the test setup becomes too complex, we will be happy to help you achieve conformity with the standard.
We will also carry out a toxicological evaluation of your devices.
Contact us via our free micro-consulting service.