Medical Device Software, IEC 62304 and FDA requirements
In this section we cover all aspects related to medical device software. You will find hints on how to effectively and efficiently fulfill the requirements by IEC 62304 and the FDA. Articles cover the entire software life cycle and respective regulations.
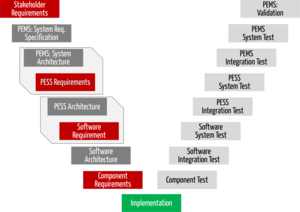
Software Lifecycle
The development process describes how, and in which sequence the involved roles (such as requirements engineers, programmers, architects, testers, etc.) transform inputs into outputs (e.g. plans, architectural design charts, software / code and other documentation).
Read more about software life cycle in general and about agile software development.
Read more about software life cycle activities:
SOUP – Software of Unknown Provenance
SOUP is an acronym for Software of Unknown Provenance. The IEC 62304 defines a SOUP as a software component,
"Which is already developed and widely available, and that has not been designed to be integrated into the MEDICAL DEVICE (also known as" Off-The-Shelf Software"), or previously developed software, not available for the adequate records for Development PROCESS. "
Mobile Medical Apps

Mobile Medical Apps, also called the Medical Apps, are applications for mobile devices such as smartphones or tablets that support medical staff or patients in the diagnosis, treatment or monitoring of a disease or injury more..