How Do You Write a Good Definition?
The importance of good definitions is best seen when there is a misunderstanding. In the worst case, a legal dispute, definitions determine whether there is liability or even whether a criminal offense has been committed. Unclear definitions, especially in legal texts, increase legal uncertainty.
This article shows you one way to write good definitions and to evaluate the quality of existing definitions. We also provide you with a checklist you can use to easily check the quality of definitions. This will allow you to communicate effectively and achieve clarity and certainty with regard to legal issues.
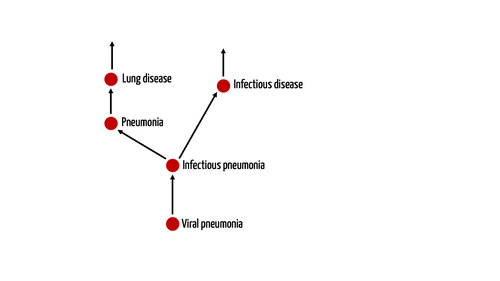
1. Definition of the term “definition”
Let’s start with the definition of the term “definition”:
Definition: Definition
A definition is a statement or rule that establishes the meaning of a concept and sets the boundaries for what does and does not belong to that concept.
Note:
In the context of this article, we use the terms “concept” and “term” synonymously. A concept can be an abstract term such as “satisfaction” or a term that describes an object such as “medical device.”
2. Writing good definitions
Definitions are usually written based on the following formula:
Term to be defined = generic term + limiting characteristics
Example: The MDR defines an invasive device as follows:
‘invasive device’ means any device which, in whole or in part, penetrates inside the body, either through a body orifice or through the surface of the body
“Device” is the generic term here. The limiting characteristic is the specification that, in order to belong to the class of invasive devices, this device must, in whole or in part, penetrate inside the body, either through the surface of the body or a body orifice.
We evaluate this definition below.
If you want to write a good definition, you should follow the steps below:
Step 1: Find your generic term(s)
As a general rule, a definition should use only one generic term. However, that’s not always the case, as the following example shows:
‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article... [limiting characteristics]
It is important to find a generic term that is as accurate as possible. In a taxonomy, for example, choose a term that is as deep in the hierarchical classification system as possible.
For example, it would not be ideal to define appendicitis as a disease. It would be better to call it an inflammation or a disease of the digestive system, as the ICD-10 classification does.
This example also shows that a term can fall into the classes of several generic terms (in this case, inflammation and disease of the digestive tract). You may already know this from polyhierarchical classification systems, such as Snomed.
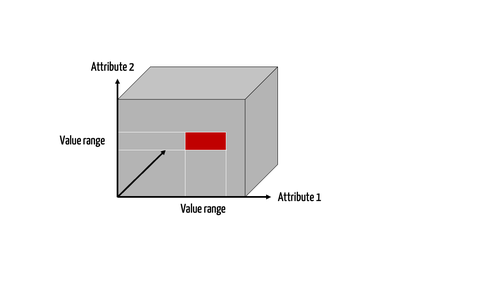
Step 2: Define the limiting characteristics
This also requires set theory: in this case, a found set is narrowed down by limiting characteristics. One or more attributes are used to do this.
These attributes in turn can be linked together by combinations of “and” and “or.” The above example of invasive devices uses “or” as the conjunction:
which, in whole or in part, penetrates inside the body, either through a body orifice or through the surface of the body”
Therefore, an invasive device is a device that has one or more of the following characteristics:
- The device can penetrate inside the body in whole through the surface of the body
- The device can penetrate inside the body in part through the surface of the body
- The device can penetrate inside the body in whole through a body orifice
- The device can penetrate inside the body in part through a body orifice
Strictly speaking, the limiting is done in two steps:
- The attribute is set and, therefore, the dimensions limited
- The value or values of the respective attribute in each respective dimension is/are set
In the example above, there are two attributes each with two values:
- The place of penetration: “body orifice” or “surface of the body”
The completeness of the penetration: “in whole” or “in part”
Step 3: Review the definition using the checklist
Lastly, you should use the following checklist to review the new definition:
- The definition defines only one term.
- The definition is not a tautology, i.e., it does not contain any circular references, such as “a mammal is an animal that suckles its young” or “a body of water [is] an above-ground body of water, groundwater and the sea” (Art. 330d German Strafgesetzbuch paragraph 1 no. 1).
- The definition only uses defined terms (e.g., generic terms).
- There are no circular references to other terms used.
- The definition only consists of one or more generic terms and one or more limiting characteristics.
- The generic terms are as specific as possible.
- The definition does not contain any explanations, justifications or examples. These belong to the notes.
- The attributes are atomic, they cannot be broken down into smaller units.
- The attributes are orthogonal and, therefore, “free of overlaps.” This also ensures that the definition uses as few attributes as possible.
- The attributes are simple and binary.
- The attributes do not extend the generic term, which is the case in the following example: “Invasive devices are medical devices used to treat animals.” In Europe, medical devices are only used to treat human patients.
- Attributes must be “pure” and must not contain explanations, examples and justifications.
- The references in a definition must be absolutely clear. For example, the definition of the term “invasive device” could trigger a discussion of whether “in whole or in part” refers only to the body orifices or also to the surface of the body.
DOWNLOAD CHECKLIST
“Writing good definitions” checklist as a download
3. Definitions: examples from the MDR and IVDR
The MDR and IVDR contain numerous definitions (71 in the MDR). The quality of most of the definitions stands up when scrutinized using the checklist. But there are also some definitions that raise questions:
Definition | Comment |
‘recall’ means any measure aimed at achieving the return of a device that has already been made available to the end user | Does this make a manufacturer's request for a device to be returned because it has not been paid for count as a recall? It is possible that the generic term “any measure” is not correct. |
‘common specifications’ means a set of technical and/or clinical requirements, other than a standard, that provides a means of complying with the legal obligations applicable to a device, process or system | A lot of best practices that are recognized and used by authorities and notified bodies (e.g., the Johner Institute's AI guidelines) contain requirements that facilitate compliance with obligations and are not standards but are not “common specifications.” Both the generic term and the attributes are inappropriate. A better generic term would be “a set of technical or/and clinical requirements published by the EU Commission, ...” |
‘device deficiency’ means any inadequacy in the identity, quality, durability, reliability, safety or performance of an investigational device, including malfunction, use errors or inadequacy in information supplied by the manufacturer | In this case, the generic term is probably incomplete and should read “inadequacy of a device with regard to...” Or, at least, that seems the obvious improvement. |
4. Why the law has a tough time defining technology terms
The fact that readers often despair of the definitions in legislation is not always due to the essential quality of these definitions. The difficulty is often the specific requirements that the law places on definitions. These requirements often don’t keep up with fields that change quickly. And technology-related industries, such as the medical technology sector, are often among these fields.
a) As specific as possible
Legal regulations must be sufficiently well defined. This means that definitions must not leave too much room for interpretation. This is the only way of ensuring legal certainty, i.e., making sure the legal consequences are predictable.
Example: The phrase “a distance of two meters” is much more specific than “a reasonable distance.”
Correct phrasing is particularly important when it comes to threats of punishment. For example, in the Strafgesetzbuch, German law threatens particularly strict punishments. If the state is handing out punishments, citizens must be able to find out exactly what behavior can lead to punishment. However, it can be important to be particularly specific in legal provisions in other circumstances too.
Conversely, legal provisions must also not be interpreted beyond their actual wording. While it is possible to read between the lines in laws and regulations and interpret what was presumably meant, this interpretation must not go beyond what is actually written. If a definition says “a distance of two meters,” you can't just read into this “a distance of four meters” just because it might seem more appropriate.
b) As far as necessary
When the reference points of the terms that the law defines change rapidly, it becomes difficult to provide specific guidance. The more specific a definition, the more rigid it usually is. This contradiction particularly affects legislation regulating industries where there are frequent innovations – for example, the medical technology industry.
So, it's a matter of squaring the circle: you need definitions that are specific enough to provide legal certainty but broad enough to adapt to changing circumstances without contradicting the wording.
c) What helps?
Drafting high quality definitions (cf. section 2) is particularly important if you want to pull off this balancing act. In addition, a lot of regulations, including the MDR, are deliberately vague in some places. These ambiguities are filled with instruments that are easier to adapt than legal provisions, for example, the common specifications. However, even these clarifications usually only help for a certain amount of time – namely until they become obsolete due to new developments.
Ultimately, therefore, there is no way around regularly reviewing legal definitions to make sure they are good enough and up-to-date, and then revising them if necessary. This ensures that a legal provision can also be applied in practice. In Germany, the Federal Constitutional Court has already ruled several times (BVerfGE 16, 130, 142; 88, 203, 309 f.; 130, 263, 302) that the legislator is obliged to update regulations.
5. Conclusion
Good definitions are essential, especially when it comes to the law. But regardless of the basic quality of the text, it is not always easy to meet all the requirements. That's why we recommend that you use our checklist as a guide. If your definition meets all the criteria it mentions, you don’t have to worry about its quality.