3D Printing in Medicine: Avoiding Regulatory Traps
If you believe Gartner's analysis, 3D printing in medicine has surpassed the “peak of unrealistic expectations”. A lot of companies are still trying to jump on the bandwagon. However, they do not have a precise idea, or know, how the law regulates 3D printing. The regulations are complex and may be different for the printer itself and for the associated software. That leads to risks in audits.
This article will give you ideas on how you can use 3D printing. It will introduce you to the relevant regulations that you need to know to be able to set up your project neatly right from the start.
1. Where is 3D printing used in medicine (or medical technology)?
a) Human spare parts
Most people, when they think of 3D printing in medicine, immediately think of “spare parts” such as
- Heart valves
- Teeth
- Joints
- Bones (after accidents or cancer)
Even more futuristic is the printing of tissue and whole organs.
b) Medical devices, tools, components
3D printing also enables us to produce medical devices, such as tools, themselves not just quickly and cost-effectively but also tailor-made for patients. Think of orthoses, dental splints and stabilizers for broken bones.
It's not always about printing a whole medical device. In fact, it is often sufficient to only manufacture parts of these devices, as is the case for spare parts or patient-specific components.
c) Templates
3D printing has already been used for years to produce patient-specific templates. Using these templates, surgeons can cut, drill and saw even more precisely.
These templates are mainly used for joint replacement operations. In radiotherapy, templates help to irradiate even more precisely near critical areas, such as the eyes or the spinal cord.
d) Simulation
There are providers who do not print replicas of the human body to use as spare parts, but instead use them for diagnostic purposes and for practicing critical operations. The company Materialise reports on how the 3D printing of a heart affected by a tumor helped model electrical signals, and simulate and prepare for the operation.
2. How is 3D printing regulated by law?
a) Europe
Is a 3D printer a medical device?
3D printers are usually not regarded as medical devices, but as a means of production. SwissMedic is clear: “The 3D printer, being hardware, is not a medical device.”
This statement is certainly correct for commercially available 3D printers. A 3D printer specifically for medical use may have to be evaluated differently if, for example, it contains software used for specific medical purposes. More on this in the next paragraph.
The associated software?
If this “embedded” software helps, for example, to determine the best geometry for a tooth, the ideal drilling positions for an implant or the optimal incision site for a surgeon (as is the case with templates), the situation is different:
Now the software and, therefore, the entire printer is used (directly) for therapeutic purposes and is thus a medical device. Rule 11 could even lead to a printer being classified in a higher category. Take a look at our article on Rule 11 and a future MDCG document.
This would also apply to standalone software with the same intended purpose. SwissMedic also comes to this conclusion in the document linked to above:
The situation is different for the software for a 3D printer. This software may be classified as a medical device depending on its functionality and intended use.
“Non-industry specific” software to control 3D printers is, in contrast, not a medical device.
Materials
Manufacturers and notified bodies have different approaches to how to evaluate printing materials (such as filaments). There are manufacturers who classify the “print result” (e.g., the tooth) as the medical device. There are also manufacturers who market the materials themselves as medical devices.
In contrast, other “material manufacturers” refer to the products simply as “medical grade”. Testing on these materials has already demonstrated that they meet the general safety and performance requirements. This testing includes biocompatibility testing.
Scanner
3D models are regularly generated by scanning an original. If the scanner manufacturer has not developed their product specifically for the medical sector, this scanner is not a medical device - instead it is, as a general rule, a means of production.
There are, however, also 3D-CAD/CAM scanners on the market, e.g., for dentistry, that are used directly on the patient and are therefore specially declared as medical devices.
The 3D printer as a means of production
If a manufacturer uses 3D printers to print medical devices, the printer is clearly part of a quality-related process. Manufacturers must manage the process in accordance with the regulatory requirements (MDD, MDR), particularly those relating to the quality management system or corresponding to the harmonized standard, ISO 13485 (e.g., chapter 7.5). This also includes the requirement to validate this process.
As software is also used in this process, the manufacturer also has to carry out a “computerized system validation” (CSV).
Special case: custom-made devices
The medical devices produced with 3D printers are often custom-made devices.
Definition: Custom-made device
“‘Custom-made device’ means any device specifically made in accordance with a written prescription of any person authorized by national law by virtue of that person's professional qualifications which gives, under that person's responsibility, specific design characteristics, and is intended for the sole use of a particular patient exclusively to meet their individual conditions and needs.”
MDR
In Annex XIII, the MDR permits the conformity assessment to be streamlined for custom-made devices. However, these simplifications do not relate to the quality management system and, therefore, do not apply to the 3D printer used.
However, the MDR restricts this apparent freedom:
However, mass-produced devices which need to be adapted to meet the specific requirements of any professional user and devices which are mass-produced by means of industrial manufacturing processes in accordance with the written prescriptions of any authorized person shall not be considered to be custom-made devices;
MDR, Article 2 (definitions)
The point of contention will therefore be whether product is industrial or not. The German Dental Association has already explained its position. It came to the following conclusion:
Since dental 3D printing is not used to produce patient-specific mass-produced devices, even if the devices are produced using industrial production processes, these medical devices are still custom-made devices according to the MDR.
The Association refers to a statement from the European Commission (Ref. Ares(2017)4450987-12/09/2017) for this assessment. This statement can lead to differences of opinion of what is meant, for example, by “manufacturer”. We are still waiting for a decision from the Supreme Court on this.
b) USA / FDA
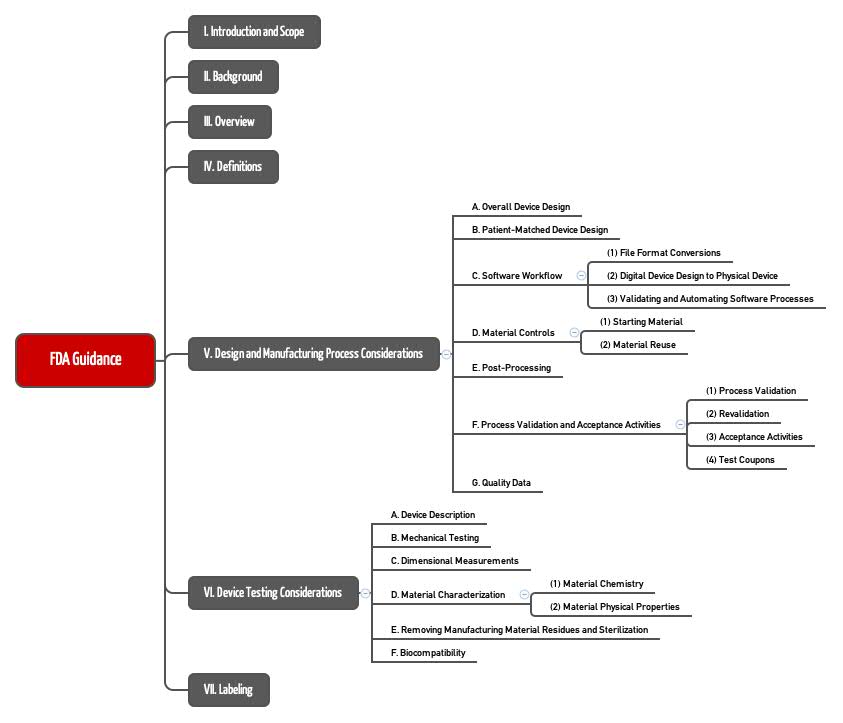
The requirements in the USA are generally comparable, but more specific. The FDA also does not generally consider 3D printers to be medical devices, but as a means of production. As a result, it has set out its requirements on “Additive Manufactured Medical Devices” in a detailed guidance document (see Fig. 1).
Fig. 1: Chapter structure of the FDA guidance document on the 3D printing of medical devices, which it calls “additive manufactured medical devices”.
The requirements apply to the entire process:
- Development
- Production / manufacture of the medical device
- Testing of the printed medical device
The FDA deals with specific aspects. For example, it talks about software:
- Errors in the file format or format conversion. The FDA proposes the additive manufacturing file format (AMF).
- Effect of taking print commands from the model, e.g., when positioning support materials
- Applicability of the guidance document to software validation
3. Conclusion
The big hype about 3D printing in medicine may have passed in some areas. But what counts is the actual application. And here, the curve is pointing steeply upwards.
There is occasionally regulatory uncertainty as to what constitutes a medical device.
In 2017, the FDA issued a guidance document, which is a very helpful guideline as to what requirements have been established for the process as a whole. This guidance document should also be used by manufacturers in Europe to analyze and control risks specific to 3D printing.
Once again, it is the software that, on the one hand, enables progress and that, on the other, is the subject of a lot of focus due to the risks that can result from software errors.