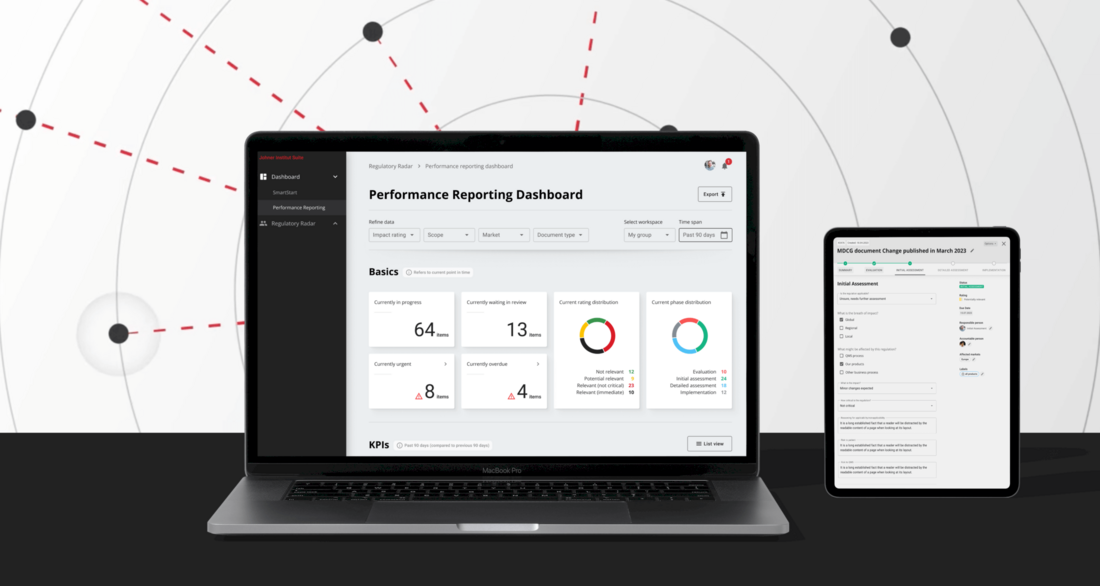
Always on track with the Regulatory Radar – for seamless compliance in the medical technology and pharmaceutical industry
In the medical technology and pharmaceutical industry, more and more regulations are being added worldwide, which are not only becoming much more complex, but are also changing more and more frequently. Keeping on top of things and keeping up with the legal requirements is not easy. Especially when resources are scarce anyway. The risk of missing important regulatory developments or noticing them too late is high and, in the worst case, can lead to significant financial harm.
7000+
medical device and pharmaceutical regulations from over 100 countries, including DINs, ENs, ISOs, FDA Acts and guidance documents, are monitored.
All
main markets are covered. This includes Europe, the United States, Brazil, Canada, China, and Japan.
50
experts from the Johner Institute are involved in the evaluation of the search results.
Save time and energy – we will keep an eye on the legal requirements
Thanks to Regulatory Radar, you can use your valuable time and resources more productively from now on - we take the continuous monitoring of legal requirements off your hands and provide you with a comprehensive overview of all changes and innovations relevant to you in a customized report. Our regulatory monitoring solution monitors over 6,000 regulations from over 100 countries daily and proactively informs you of relevant developments.
Reliable compliance monitoring: Regulatory Radar does not miss any important developments
Regulatory Radar updates you with the latest global regulations and ensures your international market success. Our solution navigates you safely through the regulatory environment and gives you the certainty that you always meet all relevant requirements. This means you run no risk of delaying the approval of your devices or suffering other financial losses due to a lack of regulatory compliance.
Regulatory knowledge on demand: Save yourself the manual search and evaluation of regulations
With our comprehensive regulatory database, you are well-equipped to quickly and easily find information on regulations in different countries and on various devices or topics. Our experts also evaluate all identified changes and innovations for you. This overview enables you to develop and adapt your regulatory strategy effectively.
Efficient regulatory monitoring
– structured, automated, and cost-saving
Regulatory Radar guarantees you a reliable and structured monitoring process of global legal requirements relevant to you. Automated monitoring using intelligent algorithms not only saves you time and money by eliminating your manual effort but also by reducing the subsequent effort required to rectify any compliance problems that arise.
Would you like to find out how our Regulatory Radar can take the pressure off you and provide reliable support for your regulatory compliance?
Book your free, non-binding meeting to gain further insights into the benefits of our solution.

