Code Verifier: Is A Smartphone Enough?
Code verifiers are optical measuring instruments that are used to check the quality of data matrix codes and barcodes used, for example, as unique device identifiers (UDIs).
Medical device manufacturers should know:
- What can go wrong when assigning and affixing these codes
- What regulatory requirements they must comply with in terms of the code quality
- How they can verify whether these requirements have been met
- How barcode and data matrix code verifiers can help them do this
- Whether smartphones can be used to verify codes
- How much a code verifier costs
1. What types of error code verifiers have to find
Error type 1: incorrect syntax / incorrect format
A common error is for the syntax encoded in the barcode or data matrix code does not meet the issuing entity’s specifications.
For example, brackets visible in plain text are included in the code. That would be a serious syntax error.
The issuing body GS1 uses GTINs for UDI-DIs. These must have a fixed length of 14 digits and must consist of the digits 0-9 only. Both of these rules must be met.
A barcode verifier or data matrix code verifier must detect these syntax errors. However, it cannot check semantics, for example, whether the expiration date is correct or the item actually exists.
Additional information
Read more on the subject of UDIshere. A series of videos in Auditgarant explains the regulatory requirements for UDIs and explains how manufacturers should define specific UDI codes for each issuing body (e.g., GS1, HIBCC).
The substrate material, e.g., the color of the substrate, can also have a negative effect on this contrast (see Fig. 2).
Error type 3: modulation, blurred edges
Even when the contrast is sufficiently high, code verifiers also have to detect when the print is not sufficiently “sharp” or when the density of the data matrix cells varies.
Pattern irregularities can be caused by fluctuating print speeds or incorrect printer configuration. In this context, “printer configuration” can refer to graphic printing, scaled PDF prints, or layout errors, etc. Barcodes can have irregular bar widths and bar positions.
Error type 5: incomplete printing
This could be caused, for example, by nozzles not working correctly in inkjet printers or heater cell malfunctions in thermal label printers. A code verifier must also reliably detect these errors and report them through error correction.
Error type 6: wrong size
The specifications of the issuing entities (GS1, HIBCC, ICCBA, IFA) also regulate the size of the codes. This applies, for example, to the so-called “module size” for data matrix codes and barcodes.
2. Regulatory requirements
a) In Europe
UDI system
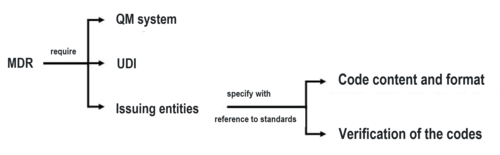
The MDR and the IVDR don’t just demand the UDI, they also require these codes to be affixed to the product in a human-readable format (e.g., in digits) and in a machine-readable format (e.g., in the form of a barcode and data matrix codes).
QM system
In the context of the QM system, the MDR and IVDR require the following in Article 10(h):
verification of the UDI assignments made in accordance with Article 27(3) to all relevant devices and ensuring consistency and validity of information provided in accordance with Article 29
MDR / IVDR Article 10(h)
The person responsible for regulatory compliance, among others, is responsible for the correct manufacture of the device, which includes the affixing of the UDI.
The person responsible for regulatory compliance shall at least be responsible for ensuring that: (a) | the conformity of the devices is appropriately checked, in accordance with the quality management system under which the devices are manufactured, before a device is released [...]
MDR / IVDR Article 15
Codes according to the specifications of the issuing entities and therefore standards
In the aforementioned Article 28(3), the MDR states:
Before placing a device [...] on the market, the manufacturer shall assign to the device [...] a UDI created in compliance with the rules of the issuing entity designated by the Commission in accordance with paragraph 2.
MDR, Article 28(3)
Therefore, the MDR (and the IVDR in exactly the same words) indirectly makes the specifications of the issuing entities (GS1, HIBCC, ICCBA, IFA) mandatory.
These specifications are available in the form of referenced standards:
Issuing entity | Referenced standards |
GS1 | ISO/IEC 15415 |
HIBICC | ISO/IEC 15416 |
ICCBA | ISO/IEC 15415 |
IFA | ISO/IEC 15415 |
In addition, the issuing entities specify other measurement parameters, such as the setting of the measurement aperture depending on the module size.
This makes standards that specify the content and format of the code and their verification (see Fig. 5) mandatory.
Measuring equipment
Code verifiers are test devices, or measuring equipment, as ISO 13485 calls them. Manufacturers must ensure that they inspect, calibrate and adjust this measuring equipment according to the requirements of this standard.
Additional information
Read this article on measuring equipment to find out which legal requirements manufacturers must comply with and what the differences are between calibration, adjustment and verification are.
b) In the USA
In the USA, 21 CFR part 820.120 requires “device labeling”. 21 CFR part 830 is more specific. In subpart B, the law references the ISO/IEC 15459 family of standards. This family of standards consists of several parts, such as the following:
- ISO/IEC 15459-2:2006, Information technology – Unique identifiers – Part 2: Registration procedures
- ISO/IEC 15459-4:2008, Information technology – Unique identifiers – Part 4: Individual items
- ISO/IEC 15459-6:2007, Information technology – Unique identifiers – Part 6: Unique identifier for product groupings
This family of standards defines the basics of automatic identification for UDIs. The GS1 specifications are based on it and define the data coding and labeling qualities that have to be complied with for codes and, therefore, also for UDI codes.
Further information can be found in the Department of Health and Human Service’s Federal Register.
3. How barcode verifiers and data matrix verifiers work
Code verifiers are generally optical test devices that capture images of the code. For this, you need to ensure defined conditions regarding:
- Camera angle
- Distance between the test device and the code
- Lighting conditions, e.g., uniform lighting
- Light source (color spectrum, measuring aperture)
- Background
The code verifiers then evaluate these images. The evaluation criteria are determined by the above standards. These standards also specify the test cases that cover all of the above error types.
Manufacturers could also check the code quality requirements (e.g., contrast ratios, size of the modules) using a measuring microscope. But these microscopes are usually more expensive, and the time and effort required for the measurement is higher than with a code verifier. The user would also need to have the expert knowledge that comes included in the verifier software.
4. Costs of barcode and data matrix code verifiers
A good code verifier costs a mid to high four figure sum. As is often the case, there are no upper limits. But with these amounts, manufacturers are getting test equipment that meets the regulatory requirements.
Leasing equipment usually doesn’t pay off. So, if you don’t have many codes that need checking, we recommend purchasing the measurement as a service. The costs for this are typically limited to a low three-figure sum.
5. Conclusion and summary
There are strict regulatory and normative requirements
While the harmonized standard system is falling apart in many areas, in the case of UDIs the standards provide specifications that are as precise as they are binding. They help avoid uncertainties during audits and authorizations.
A smartphone is unfortunately not enough
A smartphone is not a suitable barcode or data matrix code verifier:
- Smartphones do not meet regulatory requirements.
- They tempt the user to adjust the angle and distance to the code until the smartphone can read it, meaning quality deficiencies are not detected.
- Manual scanning also does not reflect the everyday situation, e.g., scanning devices during production when the distances and angles are fixed.
- In a lot of situations, the scanning process only lasts fractions of a second, but users often give a smartphone much longer reading times. This hides errors that lead to failures when there are very short read times.
It is usually worth buying a code verifier
The cost of a code verifier is manageable enough to make their purchase worthwhile. There are exceptions for purely digital products since there are no “printing problems” that a code verifier would have to detect.
Wilfried Weigelt made a substantial contribution to the writing of this article. He is a member of the DIN / ISO standards committee on automatic identification and a department manager at REA, a code verifier supplier. If you have any questions, Mr. Weigelt will be happy to help further via email.